DI Systems for Deionization
DI Systems for Deionization
Deionization refers to a specialized form of Ion Exchange. See Ion Exchange.
Deionization removes ions as does Ion Exchange. Ion exchange deionization (DI) columns use synthetic resins similar to those used in water softeners. Typically used on water that has already been prefiltered, Deionization systems use a two-stage process to remove virtually all ionic material remaining in water. That is why we call the process Deionization.
In Deionization two types of synthetic resins are used, one to remove positively charged ions (cations) and another to remove negatively charged ions (anions). Cation deionization (DI) resins ions remove cations, such as calcium, magnesium and sodium and replace them with the hydrogen (H+) ion. Anion deionization resins remove anions, such as chloride, sulfate and bicarbonate and replace them with the hydroxide (OH-) ion.
In Deionization, the displaced H+ and OH- combine to form H2O.
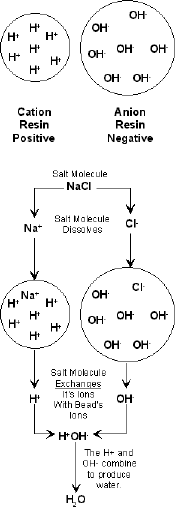
The deionization graphic at the right shows the process. In this case a salt molecule dissolves into its cation Na+ and Anion Cl-. Deionization occurs with the Na+ replaced by the Hydrogen Ion – (H+) and the Cl- replaced by the Hydroxide Ion – (OH-). Deionization occurs as the H+ and OH- combine to produce good old H2O or water.
Deionization resins have design capacities and must be regenerated and/or replaced upon exhaustion. This occurs when equilibrium between the adsorbed ions is reached. Cation deionization resins are regenerated by treatment with acid, which replenishes the sites with H+ ions. Anion deionization resins are regenerated with a strong base which replenishes (OH-) ions.
Regeneration can take place off-site with regenerated ?exchange tank? deionization tanks brought in by a service company. Please see our exchange-tank-portable-systems page for more information.
On site Deionization systems are installed complete with regeneration equipment and chemicals.
Two-Bed and Mixed-Bed Deionization
The two basic configurations of Deionization Systems are two-bed and mixed-bed.
Two-Bed deionization systems have separate tanks of cation and anion resins. In Mixed-Bed deionization systems, the anion and cation resins are blended into a single tank or vessel. Generally, mixed-bed systems will produce higher quality water with a lower total capacity than two-bed systems.
Deionization can produce extremely high-quality water in terms of dissolved ions or minerals, up to the maximum resistance of 18.3 megohms/cm. However, they do not generally remove organics and can become a breeding ground for bacteria, actually diminishing water quality where organic and microbial contamination are critical.
Failure to regenerate the deionization resins at the proper time may result in harmful salts remaining in the water or even worse, being increased in concentration. Partially exhausted deionization resin beds can increase levels of some dangerous contaminants due to the resin?s selectivity for specific ions, and may add particulates and resin fines to the deionized water.
You can look at this page to determine how long your Deionization system should last. These conversion factors show the units of Deionization which typically is Resistivity and how resistivity compares with Conductivity and TDS(Total Dissolved Solids) which are the units which typically measure the levels of dissolved impurities in your incoming water.
For Instrumentation to measure the effectiveness of your Deionization Systems, please see: Instrumentation – Myron-L Ultrameter.
We can help with your Deionization projects. When you call for assistance with your Deionization project, we will ask what are the Dissolved minerals in your incoming water. If you do not know we have a simple form you can submit to your local test laboratory to get you the right information to begin work on your Deionization system.
Please contact us. We would like to help with your Deionization project.
Related Information on this site is: